Bond Length :
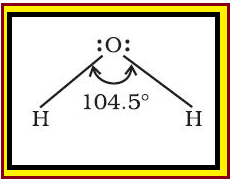
`color{red}("Definition :")` Bond length is defined as the equilibrium distance between the nuclei of two bonded atoms in a molecule.
`color{green} ✍️ color{green} mathbf("KEY CONCEPT")`
When atoms come closer to each other then attraction takes place between them and P.E. keeps on decreasing till at a particular distance and here potential energy is minimum. If the atoms are brought closer, the repulsion starts and therefore potential energy of the system begins to increase. Thus at equilibrium distance the atoms keep on vibrating about their mean positions.
● Bond lengths are measured by spectroscopic, X-ray diffraction and electron-diffraction techniques.
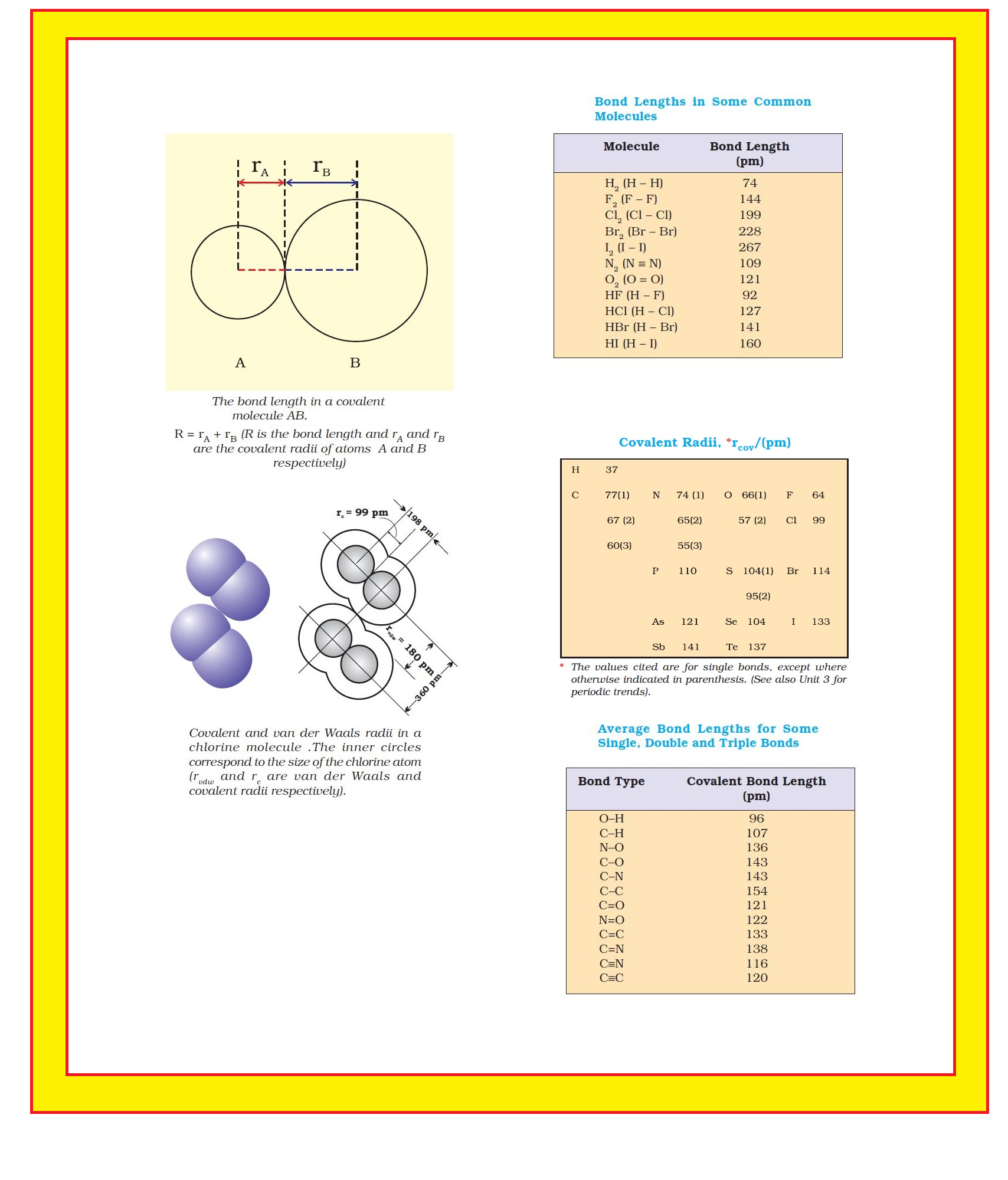
● Each atom of the bonded pair contributes to the bond length (Fig. 4.1).
● In the case of a covalent bond, the contribution from each atom is called the covalent radius of that atom.
`color{green}("Covalent Radii ")`: ● The covalent radius is measured approximately as the radius of an atom’s core which is in contact with the core of an adjacent atom in a bonded situation.
● The covalent radius is half of the distance between two similar atoms joined by a covalent bond in the same molecule.
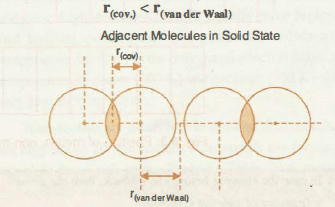
`color{green}("van der Waal's Radii" )`: ● The van der Waals radius represents the overall size of the atom which includes its valence shell in a non-bonded situation.
● The van der Waals radius is half of the distance between two similar atoms in separate molecules in a solid.
`=>` Covalent and van der Waals radii of chlorine are depicted in Fig.
`=>` Some typical average bond lengths for single, double and triple bonds are shown in Table.
`=>` Bond lengths for some common molecules are given in Table
`=>` The covalent radii of some common elements are listed in Table
•`color{red}("Factors affecting bond length ")` :
`=>` BOND LENGTH `prop` SIZE OF ATOMS
Eg. HI>HBr>HCl.
`=>` BOND LENGTH `prop 1/text(MULTIPLICITY)`
`color{green} ✍️ color{green} mathbf("KEY CONCEPT")`
When atoms come closer to each other then attraction takes place between them and P.E. keeps on decreasing till at a particular distance and here potential energy is minimum. If the atoms are brought closer, the repulsion starts and therefore potential energy of the system begins to increase. Thus at equilibrium distance the atoms keep on vibrating about their mean positions.
● Bond lengths are measured by spectroscopic, X-ray diffraction and electron-diffraction techniques.
● Each atom of the bonded pair contributes to the bond length (Fig. 4.1).
● In the case of a covalent bond, the contribution from each atom is called the covalent radius of that atom.
`color{green}("Covalent Radii ")`: ● The covalent radius is measured approximately as the radius of an atom’s core which is in contact with the core of an adjacent atom in a bonded situation.
● The covalent radius is half of the distance between two similar atoms joined by a covalent bond in the same molecule.
`color{green}("van der Waal's Radii" )`: ● The van der Waals radius represents the overall size of the atom which includes its valence shell in a non-bonded situation.
● The van der Waals radius is half of the distance between two similar atoms in separate molecules in a solid.
`=>` Covalent and van der Waals radii of chlorine are depicted in Fig.
`=>` Some typical average bond lengths for single, double and triple bonds are shown in Table.
`=>` Bond lengths for some common molecules are given in Table
`=>` The covalent radii of some common elements are listed in Table
•`color{red}("Factors affecting bond length ")` :
`=>` BOND LENGTH `prop` SIZE OF ATOMS
Eg. HI>HBr>HCl.
`=>` BOND LENGTH `prop 1/text(MULTIPLICITY)`